Safely in the future
Stricter regulatory requirements, increasing international competition, ongoing shortage of skilled workers, new customer requirements, rapid technological change: in medical technology you are confronted with numerous challenges every day.
The ERP industry software from KUMAVISION supports you not only in making your day-to-day business more efficient, but also in successfully driving growth and thus the future security of your company.
- Compliant at all times: ISO 9001-certified, validatable Validable according to MDR and ISO 13485, standard-compliant release management
- Best Practice: 350+ industry-specific functions and workflows
- Integrated solution: Inventory management, production, warehouse & logistics, QS/QM, service and financial accounting in one software
- Future-proof: The technology platform Microsoft Dynamics 365 enables easy expansion with CRM solutions (sales, service, marketing), business intelligence, Office and IoT - without any data silos.
NEW: The KUMAVISION quality platform
ERP software, ECM/DMS and QM processes on one platform
- Digitalization of the entire product life cycle
- Secure mapping of QM, QS and QK
- Ready for MDR, ISO 13485, FDA, UDI
- Digital storage of all documents, receipts, evidence and certificates
- Full integration with Microsoft 365, e.g. B. Outlook and Teams
KUMAVISION: The gold standard for medical technology
More efficiency
More efficiency
Automated processes relieve your employees of time-consuming routine tasks. Complete all tasks in one software - without any data silos. Connect customers and suppliers electronically.
More best practice
More best practice
Use the gold standard for medical technology. More than 300 best-practice processes avoid time-consuming and costly individual developments and thus enable a short time-to-value.
More transparency
More transparency
Control your company with key figures in real time. Stay informed at all times. Recognize impending bottlenecks in advance so that you can take action in good time.
More compliance
More compliance
Whether ISO 13485, MDR or FDA: With ERP industry software from KUMAVISION, you can reliably map normative requirements, relieve your employees of time-consuming routine tasks and are optimally prepared for audits.
More future
More future
Open up new markets with new business models such as pay-per-use or predictive maintenance. Respond flexibly to changing customer requirements. Get anytime access to leading Microsoft technology.
More possibilities
More possibilities
DMS solutions including workflow management, CRM for service, sales and marketing, business intelligence, mobile apps, IoT ... With KUMAVISION, all options are open to you. Fast, easy, industry-oriented.
Just everything. Everything easy.
- Validation Safely map regulatory requirements
- Internet of Things (IoT) Ready for new business models
- UDI Seamless ERP integration
- DMS integration Control and manage documents
- MDR Compliance as standard
- Field Service Mobile field service connection
- Traceability Serial and batch number tracking
- CRM solutions 360 degree view of contacts and companies
- Business intelligence Better data, better decisions
- Compliance support (ISO, MDR, GMP or FDA)
- Graded user authorization concept
- Four eyes principle with approval workflows
- Audit Trail (complete tracking of changes)
- Quality Management (QM)
- Risk and Complaint Management (FMEA)
- Integrated, audit-proof document management system (DMS)
- Supplier Evaluation
- Tracing of serial and batch numbers (traceability)
- Unique Device Identification (records, label printing, upload in GUDID)
- Electronic device file (Medical Device File)
- Validation of KUMAVISION software and development processes
- Transparent manufacturing
- QS/QM documentation
- Pay per use
- Predictive Maintenance
- Monitoring of transport and storage conditions
The industry software manages and generates all UDI-relevant information such as Device Identifier (DI) and Production Identifier (PI). While the DI is a fixed, ISO-based code for identifying the article and the manufacturer, variable data such as serial and batch numbers or the expiry date are stored in the PI by the ERP system.
UDI-compliant labels can be output directly from the industry software via NiceLabel. In addition to plain text, machine-readable 1D and 2D barcodes in the GS1 and HIBC standard are supported.
In addition, the upload to the GUDID database (Global Unique Device Identification Database) is possible directly from the ERP software.
- Legally binding marking
- Automated Unique Device Identifier (UDI) workflows
- Flexible label printing or direct marking
- Integrated GUDID upload
- No double data maintenance, no media breaks
The document management system (DMS), which is seamlessly integrated into the ERP software, supports and relieves companies when dealing with documents and data of all kinds. Documents can be easily classified using a matrix and directly assigned to products or product lines. The entire product life cycle is taken into account and mapped in the DMS.
The documents required for audits can be made available practically at the touch of a button. In addition, KUMAVISION will offer automated uploading to online portals for remote audits and approvals.
- DMS as a single point of view for all documents in the company
- Flexible workflows for approval processes, updating and distribution of documents in all areas of the company
- System-controlled administration of drawings, approval documents, certificates of conformity, proof of suppliers, certificates and operating instructions
- Ensuring traceable assignment down to component level over the entire product life cycle in the DMS
- Management of test orders, visit reports, proof of repairs or documentation as part of FMEA and CAPA measures
- Automated output, e.g. B. Delivery note including proof of conformity and QS test results
The numerous compliance and QS-relevant functions collect all the necessary data in a central system. Intelligent assistants safely guide the user through the process and data acquisition, automated workflows relieve the user of time-consuming routine tasks.
The digital master file (Device Master Record/Medical Device File) collects all documents for the respective product via the DMS integrated in the industry software.
- Digital master file including audit-proof filing and audit trail
- Administration and monitoring of approvals
- Automatic reminder before expiry date
- Simplified coordination of office and field service
- Digital documentation including signature
- Full access to customer and device history
- Direct initiation of follow-up processes (ordering of spare parts, delivery of replacement devices, etc.)
- Efficient resource planning
- Offline-enabled apps for tablets and smartphones
- Mobile recording of working hours
Serial and batch numbers are managed or generated by the ERP system. Complex scenarios such as "device in device" can also be mapped if, for example, a device contains several components that require serial numbers. Traceability when replacing components as part of repairs
The optional use of scanners accelerates the recording of batch and serial numbers and increases the process quality in production as well as incoming and outgoing goods. In addition to labels (NiceLabel, Bartender) and delivery notes, etc., the control of solutions for direct marking is also possible during output.
- Central retrieval of all customer interactions
- Control and evaluation of all sales activities
- Simple target group determination for marketing campaigns
- Control of service activities in the field
- Management of internal training and certifications
- Planning, implementation and evaluation of events of all kinds
KUMAVISION offers you business intelligence solutions based on Microsoft that are precisely tailored to the requirements of the medical technology industry Power BI. Industry-specific key figures, reports and evaluations are already included.
Companies can quickly start with a business intelligence solution and expand or adapt it individually.
60% more sales without additional staff

How the Swiss medical device manufacturer SQ Products AG increases efficiency and growth with ERP industry software from KUMAVISION
"Last year we increased our sales by 60%, and sales are expected to increase further this year. The KUMAVISION industry software has proven to be an excellent solution for all of our needs: in the areas of supply chain (purchasing, production planning, production control, shipping processing ) the growth took place without additional staff. KUMAVISION has set up a tailor-made system for us that SQ Products AG will enjoy for a long time to come."
Stephan Locher, IT manager, SQ Products AG
For all areas of the company, for all requirements
Every business area has different requirements for the ERP software. The ERP industry solution from KUMAVISION is not only perfectly tailored to the requirements of the regulated environment, but also brings individual functions, views and evaluations for every role in the company.
- Visualization of all relevant key figures for corporate management
- Convenient reporting at the push of a button
- Increased efficiency through automation
- Mapping of MDR, ISO 13485, UDI, FDA, ...
- Easy integration of CRM and DMS solutions
- Future security through Microsoft technology
- Optimized production planning and control (PPS)
- Single and multi-level production orders
- Parts lists with version management
- Manufacturing with serial numbers
- Batch production
- Traceability across all manufacturing steps
- External production with provision including sterilization
- ISO, MDR, GMP, UDI/EUDAMED and FDA support
- Multi-level test plans and integrated test equipment management
- Serial and batch number tracking
- Continuous traceability (traceability)
- Dedicated access and change rights management
- Multi-stage approval processes and the four-eyes principle
- Risk and Complaint Management (FMEA)
- Audit Trail
- Automated supplier evaluation
- Preparation of cost estimates for internal and external repairs
- Automatic repair pricing
- Processing of repair contracts
- Working with repair BOMs (customer / process dependent)
- Illustration of repair bridges
- Complaint / complaint management with integrated action control
- Administration of the service workshop and external processors
- Technical safety control (STK) and maintenance management
- Offline service app for efficient collaboration between field and office staff
- EDI tool for intelligent data exchange with business partners and e-commerce portals (optional)
- Commission management
- blanket orders
- Order entry via customer history and reorder lists
- Clear sales cockpit
- Margin check
- Approval workflows
- Automated order proposals
- Framework agreements
- Industry-specific pricing
- Optimized order proposal
- Invoice verification and delivery reminders
- Simple return management
- Clear credit management / debit notes
- Integrated supplier evaluation
- Order confirmation management
- Fully integrated and certified financial accounting
- Central evaluation of all business processes
- Inventory evaluation with automatisms for devaluation
- Sales evaluation at the time of delivery
- Integrated cost center and cost unit accounting
- Variable illustration of tax requirements
- Reading of account statements and advices
- consolidation
- XBRL (Extensible Business Reporting Language)
- Reminder and interest system
- Domestic and foreign payments
- IFRS postings
- Work-in-progress assessments
- Asset Accounting
- UDI-compliant labeling
- Barcode-supported warehouse management
- Mapping of different storage zones and locations with different storage strategies such as free choice of storage location
- External and consignment warehouse including external property management
- Different storage and retrieval strategies (e.g. First Expired First Out (FEFO) principle, 2-stage picking)
- Quality assurance through test orders in incoming goods including test equipment management
- Connection of shipping service providers such as DHL, TOF, UPS, TNT, DPD and GLS as well as integrated parcel tracking
- Deadline inventory and permanent inventory
Medical technology 2025 – success strategies for the future
In this white paper you will learn ...
- How to successfully master the challenges in the regulated environment
- Which possibilities for automation and increase in efficiency a modern industry software for medical technology opens up for you
- How to implement the requirements of MDR, ISO 13485 and UDI in a compliant and time-saving manner
- What role business software plays in the development of new business models for the medical technology industry
Current topics
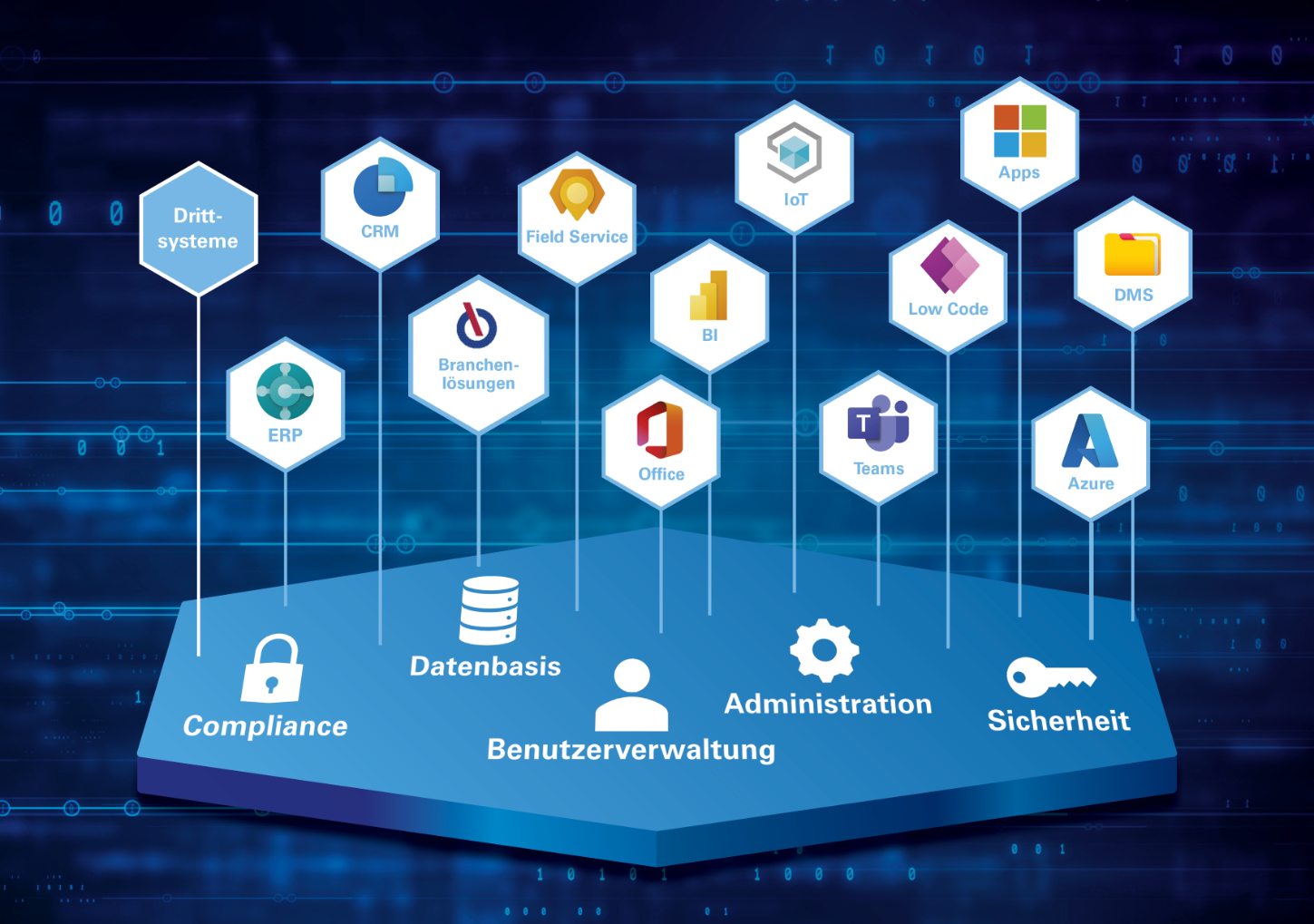
Microsoft Dynamics 365: The world's leading technology platform
With Microsoft Dynamics 365 adapt your IT landscape quickly and easily to your individual requirements. Respond promptly to changes and introduce new solutions thanks to the Cloud in a short time. At the same time, you benefit from Microsoft's annual development budget of 20 billion US dollars, giving you direct access to new technologies and tools.
Cloud is not the same Cloud!
Public Cloud
Public Cloud
Access Software-as-a-Service (SaaS) offerings that are standardized from the Cloud available for many industries. Your advantage: Fast deployment, attractive costs, easy scalability and maximum flexibility.
Private Cloud
Private Cloud
Use one Cloud-Environment set up and operated exclusively for your business. Your advantage: tailor-made Cloud-Services that exactly reflect your individual requirements and are provided at a location of your choice.
Hybrid solutions
Hybrid solutions
Combine one or more Public Clouds with a private Cloud or a local installation (on-premises). Your advantage: You determine the time and scope of your Cloud-Migrate and meet the strictest compliance requirements for sensitive data.